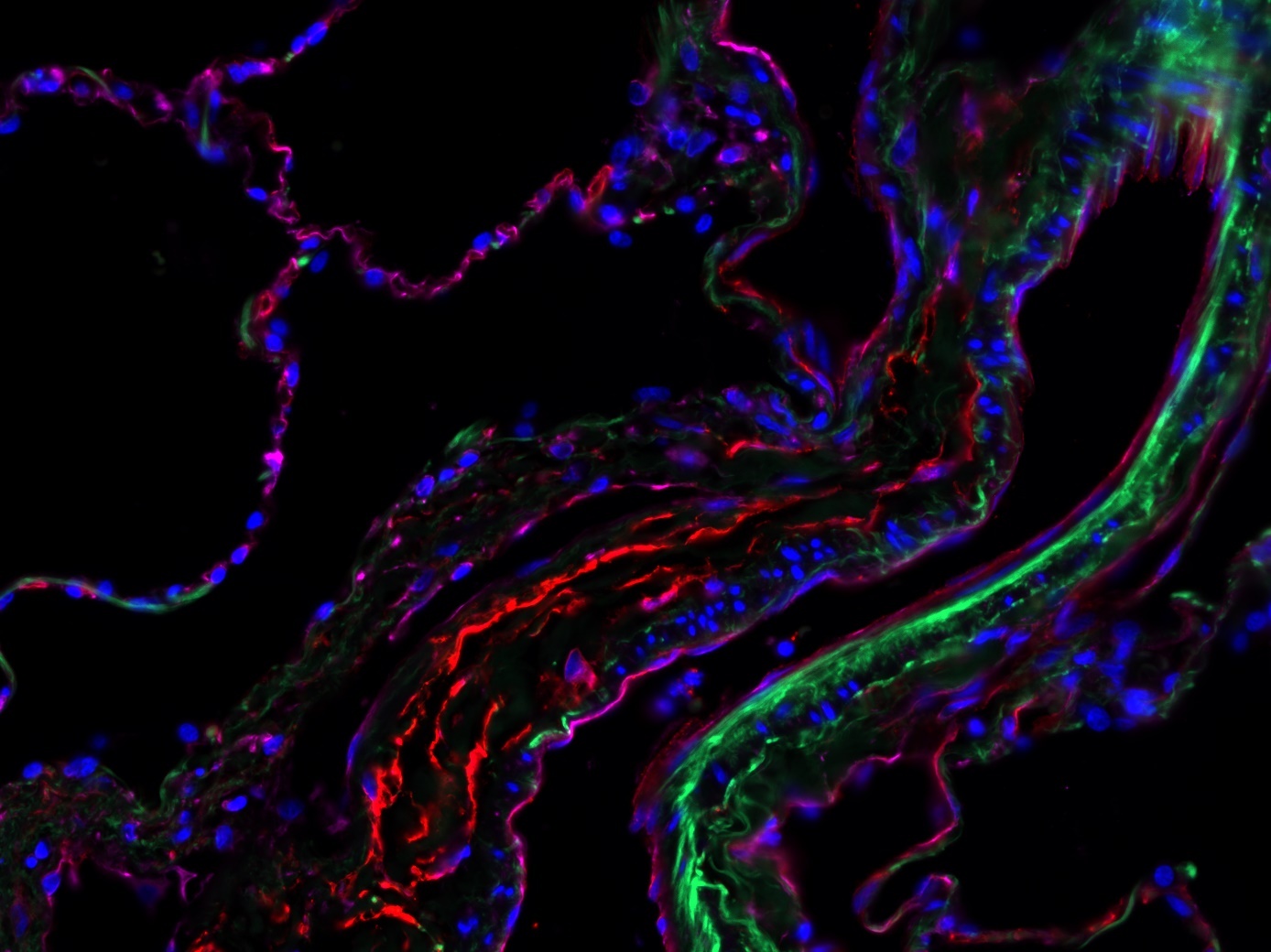
Laboratory Klein – Vascular remodeling and normal tissue protection
Research focus
Vascular function/dysfunction as a function of ionizing radiation
The aim of radiotherapy is to reduce or eliminate the tumor burden as much as possible while sparing normal tissue from radiation damage. However, the high intrinsic sensitivity of certain normal tissues often limits the application of curative radiation doses, so that local recurrence and distant metastases can occur.
Ionizing radiation leads to DNA damage, which primarily damages and kills rapidly proliferating tumour cells. In addition, it has been shown in recent years that blood vessels play a decisive role in the radiation response and that blood vessels could therefore be a promising therapeutic target. The radiosensitivity of blood vessels appears to depend crucially on the morphology and therefore the size of the blood vessel.
The research work of this junior research group focuses on the importance of endothelial cells and therapy-induced vascular remodeling for radiosensitivity. The aim is to identify key signalling molecules and possible therapeutic strategies that on the one hand radiosensitize the vessels or endothelial cells in the tumour (to improve the efficiency of radiotherapy) and on the other hand protect the endothelial cells in normal tissue during irradiation (to minimize the risk of radiation-induced normal tissue damage).
With regard to the radiosensitization of tumor endothelial cells, the focus is on the membrane protein caveolin 1 (Cav1). In various tumor entities and especially in prostate carcinoma, a very characteristic redistribution of caveolin can be observed: During tumor progression (indicated by the Gleason score), there is an increased expression of caveolin in the epithelium and Cav1 expression in the reactive tumor stroma decreases. The stromal vessels or endothelial cells are spared from this caveolin redistribution.
Interestingly, caveolin1-deficient endothelial cells in the tumor promoted tumor growth because the newly formed vessels have an angiogenic, less stabilized phenotype and a higher vascular density in the tumor. In contrast, there was an increased growth delay after irradiation of the tumor, which was due to an increased apoptosis rate of Cav1-deficient endothelial cells. This leads to the hypothesis that a reduction of endothelial caveolin could improve the radiation response in the tumor. Detailed investigations into the molecular and cellular mechanisms of caveolin1-induced changes in the radiosensitivity of tumor and normal tissue cells are the subject of current studies in subproject 12 of the recently extended Essen Research Training Group GRK1739.
Apl. Prof. Dr. rer. nat.
Diana Klein
Laborleiterin
(Head of laboratory)
Protection of normal tissue damage using mesenchymal stem cell therapy
The application of a curative radiation dose in tumor therapy is often hampered by the high intrinsic sensitivity of the surrounding normal tissue. For example, inflammatory and fibrotic changes in the lungs in particular are observed as dose-limiting side effects after thoracic irradiation. No causal radioprotective treatment is currently available. Current research efforts are therefore aimed at protecting normal tissue during radiotherapy in order to achieve a possible increase in the radiation dose.
Vascular endothelial damage following radiotherapy is likely to be a key event in the pathogenesis of radiation-induced normal tissue damage, and we are currently investigating the extent to which therapy with multipotent mesenchymal stem cells (MPSCs) can counteract radiation-induced vascular damage and reduce associated side effects (pneumonitis, fibrosis). In general, tissue-specific stem cells differentiate predominantly to the tissue type from which they originate; therefore, a central working hypothesis is that the therapeutic application of vascular-resident multipotent stem cells of mesenchymal nature could be particularly well suited to favorably influence pathological processes in irradiated blood vessels.
Vascular-resident MPSCs could be of particular interest for autologous stem cell therapy in humans, as comparably more cells can be isolated from small vessel pieces just a few centimetres long than from bone marrow. The removal of vessel pieces from the saphenous vein or the radial artery, for example, would be an easy procedure to perform. Previous experience with vascular-resident stem cells also shows that they can be isolated relatively easily from the vessel wall and expanded in culture in order to obtain a sufficient quantity of cells for a possible therapeutic application.
Team

Maja Buchholzki
Medizinische Doktorandin
(Medical doctoral student)

Luca Buttler
Studentische Hilfskraft
(Student assistent)

Lea Cremer
Bachelor Studentin
(Bachelor student)

Vaanilaa Ketheeswaranathan
Wissenschaftl. Doktorandin
(Scientific doctoral student)

Linus Korten
Technischer Mitarbeiter
(Technical assistent)

Fatma Zehra Sevendik
Studentische Hilfskraft
(Student assistent)

Lisa Stasch
Wissenschaftl. Doktorandin
(Scientific doctoral student)