NG Wirsdörfer –
Immunmodulation
Research focus
Radiation-induced immune changes in normal and tumor tissue
Radiotherapy is an integral part of standard treatment for various neoplasms. On the one hand, treatment is limited by intrinsic tumor resistance and by metabolic and microenvironment-induced adaptive resistance of the tumor. On the other hand, radiation-induced normal tissue toxicity and immune-related undesirable side effects limit the course of therapy, so that ultimately different treatment successes are achieved in the patients. These limitations restrict the therapeutic window, which leads to suboptimal local control, metastasis formation and a reduced quality of life.
The research work of the junior research group “Immunomodulation” aims to elucidate the interaction between the damage to the tumor or normal tissue as a result of radiation, the activation of the innate and adapted immune system, and the development of adverse side effects.
The focus of the junior research group is on the mechanisms underlying the radiation-induced toxicity of normal lung tissue. The aim is to define new therapeutic targets with different effects in tumor and normal tissue in order to develop effective treatments that protect normal tissue without compromising tumor control. In addition, the group pursues translational research approaches by investigating the risk of new combinatorial radiotherapies with a focus on immunotherapeutics.
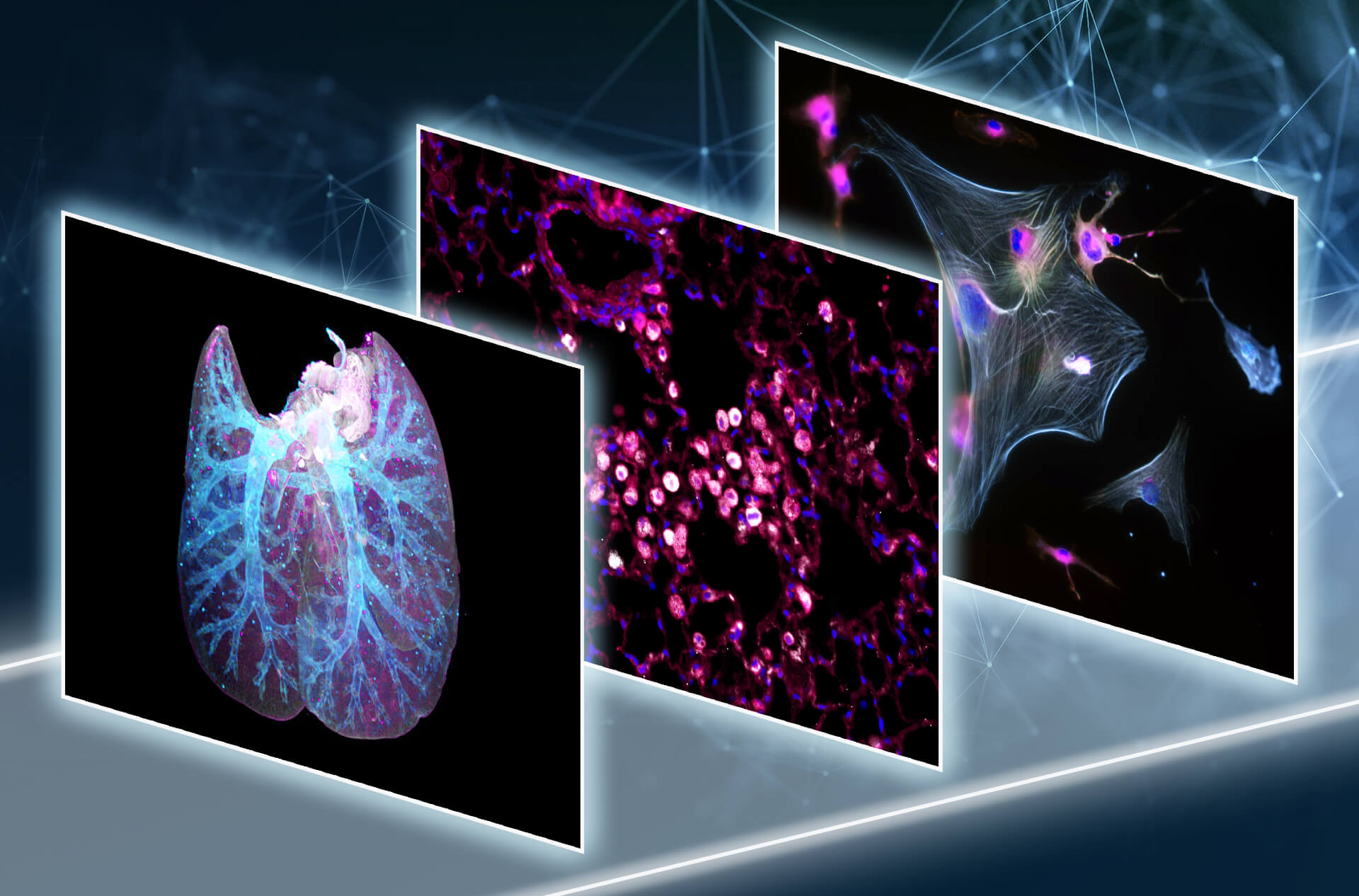
This involves co-clinical irradiation systems, immunophenotyping using multicolor flow cytometry and advanced imaging techniques such as PET-CT, multiplex and lightsheet microscopy as well as cytokine profiling.
Previous research has revealed pathologic acute and chronic immune modulations associated with radiation-induced side effects in the lung, e.g. local immune activation and systemic accumulation of immunosuppressive CD4+ T cell populations and increased CD39/CD73 activity. Current projects of the research group focus on the development of novel therapeutic targets to reduce radiation-induced normal tissue toxicity in NSCLC and HNSCC and on deciphering the complex immune-related side effects that may occur in the course of novel therapeutic approaches with radio(chemo)therapy and immune checkpoint inhibition. The main long-term goals of the research group are therefore to define predictive biomarkers for the risk assessment and detection of radiation-induced acute and chronic tissue damage and ultimately to identify new therapeutic targets to overcome treatment limitations

Dr. rer. nat.
Florian Wirsdörfer
Nachwuchsgruppenleiter
(Junior group leader)
Team

Diellza Dibrani
Medizinische Doktorandin
(Medical doctoral student)

Eva Gau
Technische Mitarbeiterin
(Technical assistent)

Dr. rer. nat.
Lena Gockeln
Postdoktorandin (Postdoc)

Mario Hetzel
Wissenschaftl. Doktorand
(Scientific doctoral student)

Constanze Tinz
Wissenschaftl. Doktorandin
(Scientific doctoral student)

Marija Vazgec
Studentische Hilfskraft
(Student assistent)
Own projects:
Dr. Florian Wirsdörfer (DFG Einzelprojekt WI 4702/2-1 bis 7/23; derzeit Beantragung Fortsetzung): Neuropilin-1 als Multi-Target bei strahleninduzierter Lungenfibrose
Doktorandin Irene Bocci (EU ITN THERADNET; Folgefinanzierung IFORES): Targeting pathologic macrophages to widen the therapeutic window
Medizindoktorand Alexander Ziebolz (MD-Projekt GRK2762/1 und Brigitte und Dr. Konstanze Wegener Stiftung): Rolle des Mikrobioms für Immunmodulation im Rahmen einer kombinierten Radioimmuntherapie
with Prof. Dr. Verena Jendrossek
UMESciA-Projekt: Dr. Lena Gockeln: Relevance of biological factors to outcome of radio(chemo)therapy or radio(chemo)therapy-immunotherapy combinations in advanced solid human tumors
Co-Tutelle Paris-Saclay: Doktorand Hugo Laporte: Role of the immune-endothelial cell-cell interactions in the failure to regenerate the lung after irradiation
GRK 2762/1: Doktorandenprojekt Mario Hetzel T1 (mit PD Dr. Nika Guberina): Biomarkers of pneumonitis and lung fibrosis upon thoracic RT/RCTx with or without concomitant anti-PD-L1 antibody therapy
GRK 2762/1: Doktorandenprojekt Constanze Tinz T1 (mit PD Dr. Nika Guberina): Biomarkers of pneumonitis & lung fibrosis upon thoracic RT/RCTx and anti-PD-L1 w/wo anti-CD73 combination therapy